Insights+: The US FDA New Drug Approvals in October 2022
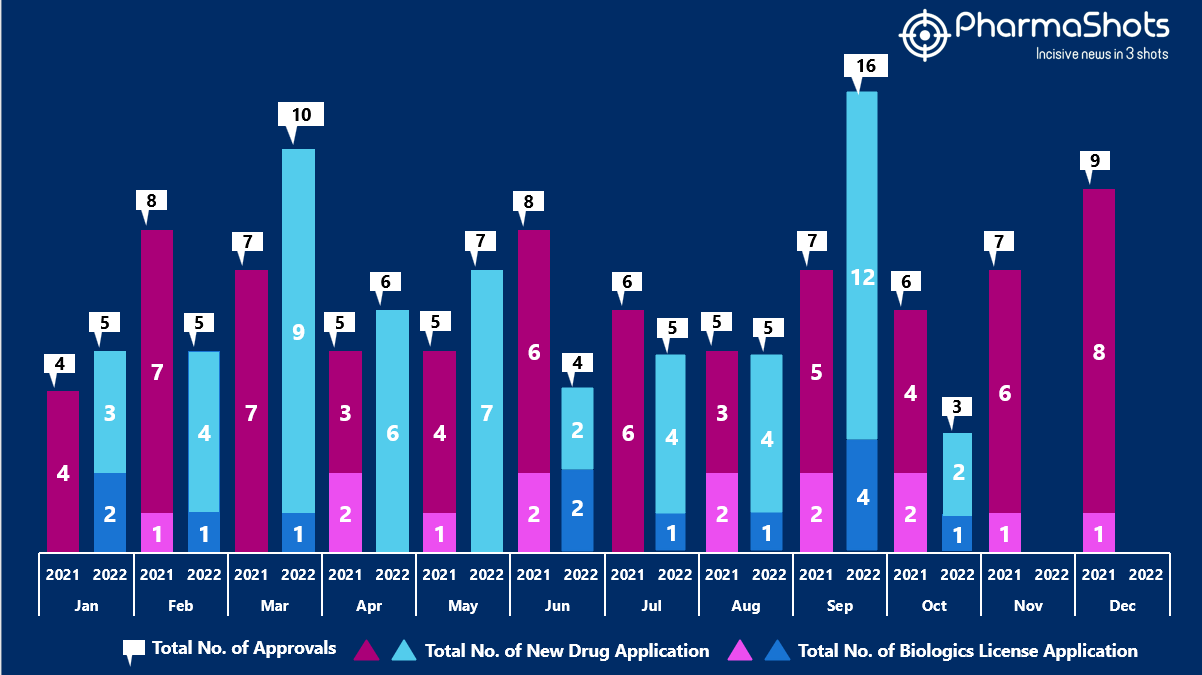
- The US FDA approved 1 NDAs and 2 BLA in October 2022, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 66 novel products in 2022
- In October 2022, the major highlights drugs were Tecvayli’s approval for relapsed or refractory multiple myeloma, Furoscix for congestion in chronic heart failure
- PharmaShots has compiled a list of a total of 3 new drugs approved by the US FDA in October 2022
Furoscix
Active ingredient: furosemide Approved: October 10, 2022
Company: scPharmaceuticals Disease: Congestion in Chronic Heart Failure
- The US FDA has approved Furoscix (80mg/10mL) for congestion due to fluid overload in adults with NYHA Class II/III chronic HF
- Furoscix (SC infusion) demonstrated 99.6% bioavailability & 8hr. urine output of 2.7L similar to IV furosemide. The P-II (AT HOME-HF) pilot study evaluates furosemide (80mg/10mL) vs SoC in 51 patients with chronic HF with congestion uncontrolled by diuresis & showed a 37% lower risk of HF hospitalization @30 days & the 2EPs showed greater declines in mean patient body weight from baseline to day 2 & improvements in pulmonary-related metrics
- Furosemide is not indicated for use in emergency situations or in patients with APE. The formulation of furosemide is administered via On-Body Infusor & the product is expected to launch in Q1’23
Imjudo
Active ingredient: tremelimumab Approved: October 25, 2022
Company: AstraZeneca Disease: Liver Cancer
- The approval was based on the P-III (HIMALAYA) trial evaluating Imjudo (300mg) + Imfinzi (1500mg, q4w) vs sorafenib in 1324 patients with HCC prior not treated with systemic therapy & not eligible for LRT at 181 centers across 16 countries
- The results showed that patients treated with the combination experienced a 22% reduction in risk of death, 31% vs 20% were still alive after 3yrs. vs the same duration of follow-up. The results were published in the NEJM with no increase in severe liver toxicity or bleeding risk
- The safety profiles were consistent with the known therapy profiles with no new safety signals. The regulatory applications are currently under review in the EU, Japan & multiple other countries for advanced liver cancer
Tecvayli
Active ingredient: teclistamab Approved: October 27, 2022
Company: Janssen Pharmaceuticals Disease: Multiple Myeloma
- The US FDA has approved Tecvayli (teclistamab-cqyv) for the treatment of adult patients with r/r MM prior received ≥4 prior lines of therapy including a proteasome inhibitor, immunomodulatory drug, and anti-CD38 mAb
- In the P-II (MajesTEC-1) trial, the therapy showed ORR (61.8%) with CR (28.2%), and the median time to first response was 1.2mos. At a median follow-up of 7.4mos., the estimated DoR rate was 90.6% @6mos.and 66.5% @9mos., 78% received ≥4 prior lines of therapy, 76% were triple-class refractory
- Tecvayli is an off-the-shelf, SC therapy for patients with incurable blood cancer with limited treatment options. The product is supplied as 30mg/3mL and 153mg/1.7mL single-dose vials
Related Post: Insights+: The US FDA New Drug Approvals in September 2022


